
Catalytic Wacker Oxidation of Tetrasubstituted Alkenes: Efficient Access to gem-Dimethyl Ketones
Congratulations! The publication of our group in Organometallics, "Catalytic Wacker Oxidation of Tetrasubstituted Alkenes: Efficient Access to gem-Dimethyl Ketones" has been published recently (Longji Wu, Wangzhen Qiu, Lihao Liao*, Bo Wang, and Xiaodan Zhao* Organometallics 2025,44, 2842-2847).

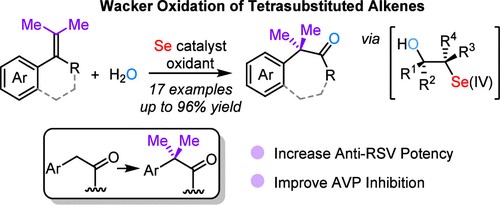
An efficient approach for the p-block selenium-catalyzed migratory Wacker oxidation of tetrasubstituted alkenes is developed. Under mild conditions, cyclic tetrasubstituted alkenes bearing aryl and geminal dimethyl groups can undergo six-to-seven or five-to-six ring expansion to give a series of ketones bearing a gem-dimethyl group via oxidative aryl migration. The method could be employed for the Wacker oxidation of acyclic tetrasubstituted alkenes as well. The obtained gem-dimethyl ketones are appealing in medicinal chemistry. It is believed that a selenenylation–deselenenylation followed by a semipinacol rearrangement process is involved in the reactions. This work provides a new strategy for the synthesis of gem-dimethyl molecules, and is complementary to the fields of p-block main group element catalysis and Wacker oxidation.
Previous page: Catalytic Asymmetric Electrophilic Chlorocarbocyclization of Alkenes Using ipso-Carbon Nucleophiles
Next page: Research Progress in Electrophilic Selenium-Catalyzed Rearrangement Reactions
