
Catalytic Asymmetric Electrophilic Chlorocarbocyclization of Alkenes Using ipso-Carbon Nucleophiles
Congratulations! The publication of our group in ACS Catal., "Catalytic Asymmetric Electrophilic Chlorocarbocyclization of Alkenes Using ipso-Carbon Nucleophiles" has been published recently (Fuming Zhong, Zongren Ye, Tianteng Zhao, Lihao Liao, Zhuofeng Ke*, and Xiaodan Zhao* ACS Catal. 2025,15, 9452-9463).

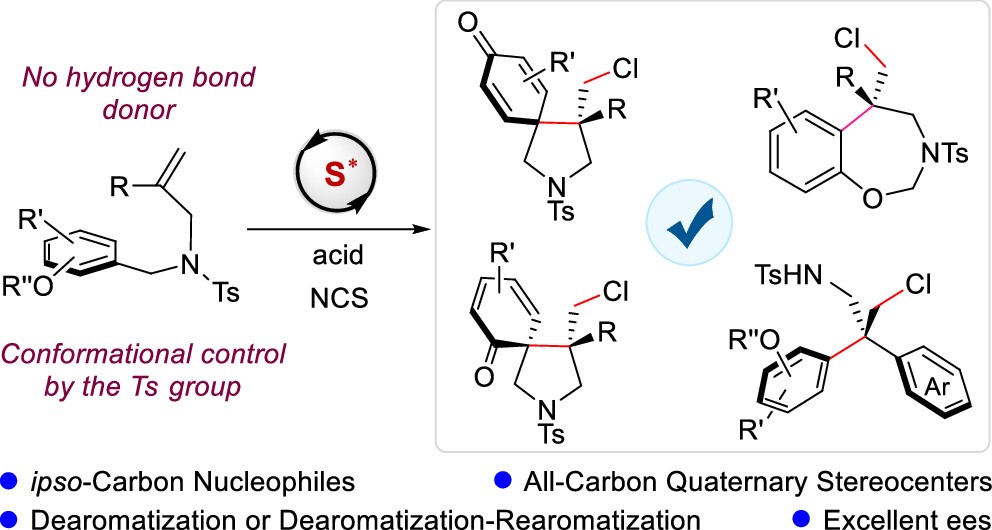
Catalytic asymmetric electrophilic difunctionalization of alkenes is a powerful strategy for accessing enantioenriched compounds. Despite significant progress in this field, achieving such transformations with ipso-carbon nucleophiles remains a formidable challenge. Herein, we report an unprecedented method for the asymmetric electrophilic difunctionalization of alkenes using ipso-carbon nucleophiles, employing N-chlorosuccinimide (NCS) as the electrophilic reagent and a bulky chiral sulfide catalyst. By utilizing 1,1-disubstituted alkenes as substrates and tethered phenol-derived aryl groups as ipso-carbon nucleophiles, a series of chiral pyrrolidines bearing up to two all-carbon quaternary stereocenters and a spirocyclohexadienone moiety were synthesized in good yields with high enantioselectivities. Furthermore, this reaction was extended to the synthesis of chiral tetrahydrobenzoxazepines and diarylethylamines via an in situ dearomatization-rearomatization process. In these transformations, stereochemical control is achieved exclusively through Lewis base complexation without the need for auxiliary binding interactions. Control experiments revealed that both the choice of electrophilic reagents and the conformational control imparted by the sulfonyl protecting group on the substrates are critical for the efficient progression of the reaction. Density functional theory (DFT) studies demonstrated an energetic preference for the selective coordination of electrophilic chlorine to one of the two lone pairs on the sulfur atom of the catalyst, forming a chiral sulfur intermediate, followed by facial recognition of the alkene π bonds.
Previous page: Catalytic Asymmetric Azidative Functionalization of Alkenes
Next page: Catalytic Wacker Oxidation of Tetrasubstituted Alkenes: Efficient Access to gem-Dimethyl Ketones
