
A catalytic thiolation/dethiolation strategy for the ring-opening of cyclopropenes to access α,β-unsaturated nitriles and carbonyl compounds
Congratulations! The publication of our group in Chin. Chem. Lett., "A catalytic thiolation/dethiolation strategy for the ring-opening of cyclopropenes to access α,β-unsaturated nitriles and carbonyl compounds" has been published recently (Quanbin Jiang, Hui Jiao, Long Jiang, and Xiaodan Zhao* Chin. Chem. Lett. 2025, DOI:10.1016/j.cclet.2025.112354).

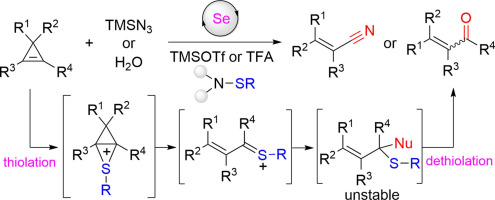
An efficient catalytic thiolation/dethiolation strategy for the ring-opening transformation of cyclopropenes is reported. The reaction occurs via the formation of a vinylogous Pummerer intermediate followed by a dethiolation Pummerer fragmentation step. A wide range of valuable products including α,β-unsaturated nitriles and carbonyl compounds can be efficiently synthesized in these reactions. The resultant products are readily transformed into other valuable molecules. The hydrogenation experiment supports the generation of the vinylogous thionium ion as a crucial intermediate in this transformation.
Previous page: Research Progress in Electrophilic Selenium-Catalyzed Rearrangement Reactions
